The nucleus of an atom mainly consists three type of particles, i.e. Electrons which are negatively charged, protones which are positively charged and neutrons which are nutral. Atom is electrically neutral because the total positive charge ( of protons) is cancelled by the total negative charge (of electrons). With the help of an example of Carbon atom, I have tried to illustrate how in any atom total number of protons = total number of electrons and that the total.

An Atom Is Electrically Neutral True Or False
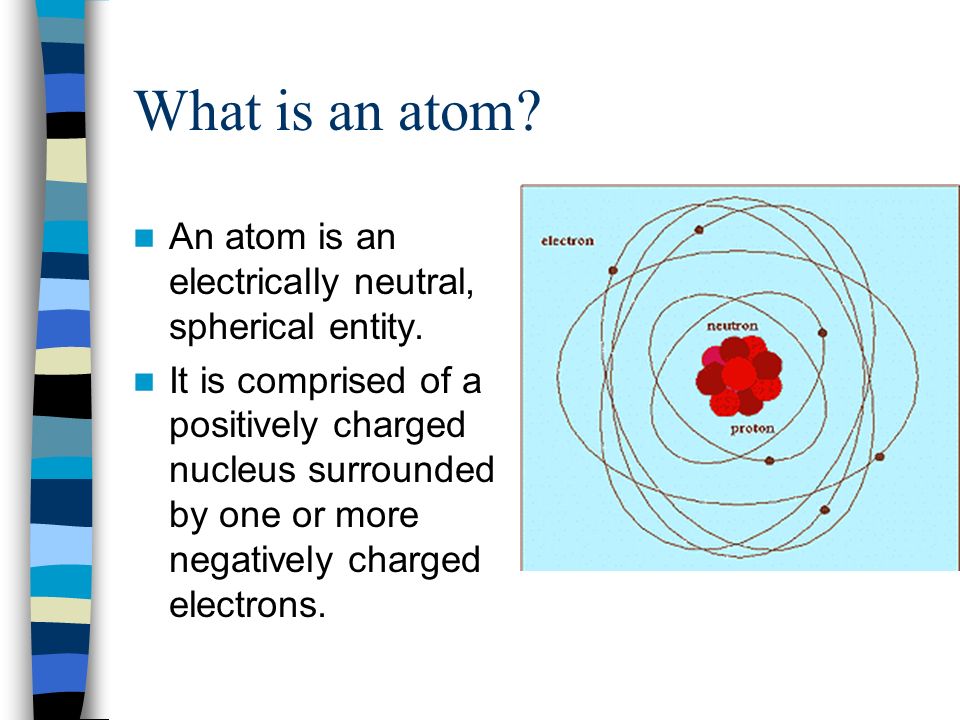
- By definition, an atom is electrically neutral (i.e. Has the same number of protons as it does electrons, plus some number of neutrons depending on the isotope). If a species were charged, it is referred to as an ion (cation for positively charged and anion for negatively charged species), also by definition.
- Atoms are electrically neutral because they have equal numbers of protons (positively charged) and electrons (negatively charged). If an atom gains or loses one or more electrons, it becomes an ion. If it gains one or more electrons, it now carries a net negative charge, and is thus 'anionic.'
- Answer 1: By definition, an atom is electrically neutral (i.e. Has the same number of protons as it does electrons, plus some number of neutrons depending on the isotope). If a species were charged, it is referred to as an ion (cation for positively charged and anion.

Atoms vs. Ions
An Atom Is Electrically Neutral. True False
Atoms are neutral; they contain the same number of protons as electrons. By definition,an ion is an electrically charged particle produced by either removing electronsfrom a neutral atom to give a positive ion or adding electrons to a neutralatom to give a negative ion. When an ion is formed, the number of protonsdoes not change. How to free up ram on windows 8.
Neutral atoms can be turned into positively charged ions by removing oneor more electrons. A neutral sodium atom, for example, contains 11 protonsand 11 electrons. By removing an electron from this atom we get a positivelycharged Na+ ion that has a net charge of +1.
Atoms that gain extra electrons become negatively charged. A neutral chlorineatom, for example, contains 17 protons and 17 electrons. By adding one moreelectron we get a negatively charged Cl- Muftakis blogspot. ion with a net charge of -1.
The gain or loss of electrons by an atom to form negative or positive ionshas an enormous impact on the chemical and physical properties of the atom.Sodium metal, for example, which consists of neutral sodium atoms, burstsinto flame when it comes in contact with water. Neutral chlorine atoms instantlycombine to form Cl2 molecules, which are so reactive that entire communities are evacuatedwhen trains carrying chlorine gas derail. Positively charged Na+ and negatively charged Cl- ions are so unreactive that we can safely take them into our bodies wheneverwe salt our food.
